Leitung: Prof. Dr. Katharina Zimmermann
Research in the Zimmermann Lab focusses on the physiological role of ion channels in the somatosensory system. This relates to delineating the function of ion channels in thermo- and nociceptive transduction and electrogenesis as well as in the related pathophysiology.
Leitung:
Interview: Preventing and treating obesity using cold sensation
Research Alumni Interview with the experienced Humboldt Fellow and soccer enthusiast Dr. Mahendra Bishnoi
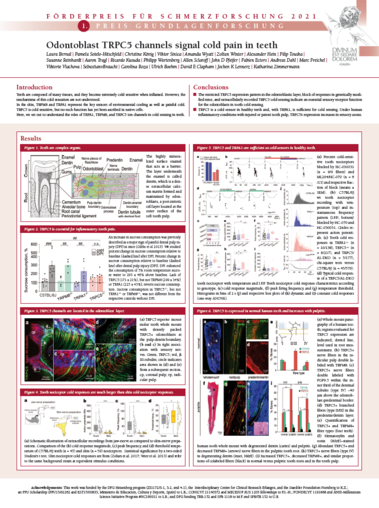
German Pain Congress "Förderpreis für Schmerzforschung 2021"
The first German Pain Research Prize was awarded on October 21st 2021 to Laura Bernal and Pamela Sotelo-Hitschfeld for our story on TRPC5 in teeth.
The poster was presented online during the German Pain Congress in Mannheim.
Most recent
Roughly, 2.4 billion people – about a third of the world’s population – have untreated caries in permanent teeth, which causes intense pain, including extreme cold sensitivity. We have now discovered the cold transduction mechanism in teeth and, our work provides the molecular targets for the treatment of cold hypersensitivity. The work is published in Science advances:
The most current theory for cold sensitivity in teeth had proposed that the fluid inside the tiny dentinal canals moves when the temperature changes and that the sensory nerves sense the direction of these fluid movements and signals tooth pain in response to hot or cold. To investigate cold nociception in teeth, we developed a novel experimental set up, which allowed us to record neural activity from intact mouse teeth in response to cold. While previous work focused on cultured sensory neurons derived from trigeminal ganglia, our preparation illustrated for the first time that the site of cold transduction in teeth lies not in the sensor nerves, but in the odontoblasts. These are the cells that build the hard substance in teeth, enamel and dentin, and our work established them for the first time as primary nociceptors. We found that TRPC5 and TRPA1 are sufficient as cold transducer channels in teeth and that TRPC5 in odontoblast is uniquely required for inflammatory tooth pain.
Media/Links
Interview Prof. Dr. Katharina Zimmermann, Mitteldeutscher Rundfunk:
https://www.mdr.de/wissen/audios/ursache-fuer-empfliche-zaehne-gefunden100.html
Further information:
https://www.bbc.com/news/health-56536300
https://www.nytimes.com/2021/03/26/science/tooth-pain-cold.html
https://www.hhmi.org/news/how-teeth-sense-the-cold
https://www.eurekalert.org/pub_releases/2021-03/mgh-rdw032621.php
https://www.inverse.com/mind-body/why-your-teeth-cant-stand-the-cold
Other important Work
The NaV1.8 sodium channel subtype functions as cold-resistant ignition on nociceptors and is also able to shape heat-resistant action potentials above 45°C. Unlike other fast-gated and low-threshold sodium channel subtypes that become inactivated by cold or hot temperatures, Nav1.8 appears resistant and increases the excitability of nociceptors at noxious cold and hot temperatures. Nav1.9, in contrast is tuned to provide a persistent current and enables nociceptors to produce action potentials in response to fast-rising temperatures and thereby to protect us from heat-induced tissue damage.
Touska F, Turnquist B, Vlachova V, Reeh PW, Leffler A, Zimmermann K. Heat-resistant action potentials require TTX-resistant sodium channels NaV1.8 and NaV1.9. J Gen Physiol 150: 1125-1144, 2018.
The voltage-gated Kv7.2/3 channels are suprathreshold amplifiers of TRPM8-mediated cold transduction. Triggered by the activation of the menthol-receptor TRPM8, the channels are progressively closed by cold, thereby increasing the cold transduction current in the nociceptive nerve endings in the skin. Menthol also proves to be a potent blocker of these important, pan-neuronal potassium channels and enhances its intrinsic TRPM8 agonist action through this effect.
The noxious cold transducer TRPA1 seems to be a central molecule in the development of pathological cold allodynia such as induced by certain fish toxins, called ciguatoxins. Ciguatoxins are synthetized bytropical dinoflagellates and accumulate in fish meat through the food chain. They cause ciguatera which frequently leads to a long-lasting cold allodynia. These potent sodium channel poisons causes membrane potential oscillations that lead to a sensitization of the temperature sensitivity of TRPA1 which turns pleasant cool into the burning painful feeling of freezing cold. TRPA1 deficient mice show significantly reduced cold allodynia, similar to Nav1.8 null mice.
In order to more closely research the contribution of individual ion channels to overall temperature sensitivity we developed a new scientific instrument which allows to phenotype thermal preference behavior without experimenter interference. This device allowed us to identify the contribution of TRPM8 and TRPA1 as synergistic in cold temperature perception and absence of both channels results in a much delayed cold avoidance behavior in mice. The device is available from Ugo Basile.
Currently we aim to understand how TRP channels shape our ability to adapt to changes in environmental temperatures, why female and male mice differ in their temperature preferendum and how peripheral cold sensing integrates with CNS processing, effectors of thermoregulation and energy metabolism . We are also interested in understanding individual susceptibility differences in cold tolerance, cold hypersensitivity and cold allodynia.
For a list of all publications go to google scholar
Members of the Team:
Guests and Visitors:
Mahendra Bishnoi, Senior Fellow of the Alexander von Humboldt Foundation (National Agri-Food Biotechnology Institute, Punjab, India) - since July 2021
Tudor Selescu (Faculty of Biology, University of Bucharest, Romania)
Collaborations:
Stephan von Hörsten, Experimentelle Medizin, Universitätsklinikum Erlangen
Andreas Kremer, Medizinische Klinik 1, Universitätsklinikum Erlangen
Alexey Ponomarenko, Institut für Physiologie und Pathophysiologie Erlangen
Jan Siemens, Institut für Pharmakologie, Universität Heidelberg
Carolina Roza, Universidad de Alcalá, Madrid, Spain
Viktorie Vlachova, Czech Academy of Sciences, Prague, Czech Republic
Aziz Moqrich, Institut de biologie du développement Marseille-Luminy (IBDML) - CNRS/ Université Aix-Marseille, Frankreich
Jeff Mogil, Pain Genetics, McGill University, Montreal, Canada
Gary Peltz, Stanford University, Palo Alto, CA, USA
Jochen Lennerz, Department of Pathology, MGH and Harvard Medical School, Boston, USA
Thiago Mattar Cunha, Pharmacology, School of Medicine of de Ribeirão Preto, University of São Paulo, Brazil
Sebastian Brauchi, Universidad Austral de Chile, Valdivia, Chile
Simon Brookes and Rochelle Peterson, Human Physiology, Flinders University, Adelaide, Australia
Makoto Tominaga, NINS, National Institute for Physiological Sciences, Okazaki, Japan
Past Members:
PhD students:
Laura Bernal, Pamela Sotelo-Hitschfeld, Filip Touska, Qiang Wang
PostDocs:
Christine König, Zoltan Winter, Aklesso Kadala, Ricardo Kusuda (FAPESP Fellow, Universidade de São Paulo, Ribeirão Preto, Brazil)
Undergraduate Students:
Aaron Tragl, Gregor Neussel, Patrick Burke, Alexander Kapp, Ziad Ahmad, Anneka Eisenblätter, Philipp Gruschwitz, Antonia Fitzek, Stefanie Eger